Current issue
Archive
About the Journal
Editorial Board
Journal Insights
Contact us
Publisher
Article Processing Charge
Editorial Policy
Peer Review Process
Publication Ethics
Guidelines
For Authors
For Reviewers
For Editors
Step-by-step Guide to Article Publishing
How to Promote Your Published Article
Reviewers
RESEARCH PAPER
Molecular approach for the detection of potential phytopathogens of strawberry plants: PCR assay using functional genes in microbial strains, and environmental samples
1
Institute of Agrophysics, Polish Academy of Sciences, Doświadczalna 4, 20-290 Lublin, Poland
Final revision date: 2025-07-29
Acceptance date: 2025-09-09
Publication date: 2025-11-04
Corresponding author
Magdalena Frąc
Department of Soil and Plant System, Institute of Agrophysics, Polish Academy of Sciences, Poland
Department of Soil and Plant System, Institute of Agrophysics, Polish Academy of Sciences, Poland
Int. Agrophys. 2026, 40(1): 39-54
HIGHLIGHTS
- Phytopathogens detection important to increase yield and product quality
- Detection method based on primers designed for selected functional genes
- Pathogens detection in contaminated environmental samples
KEYWORDS
artificially contaminated and environmental samplespotential plant pathogensstrawberrysustainable agriculturesoilplant and fruits
TOPICS
ABSTRACT
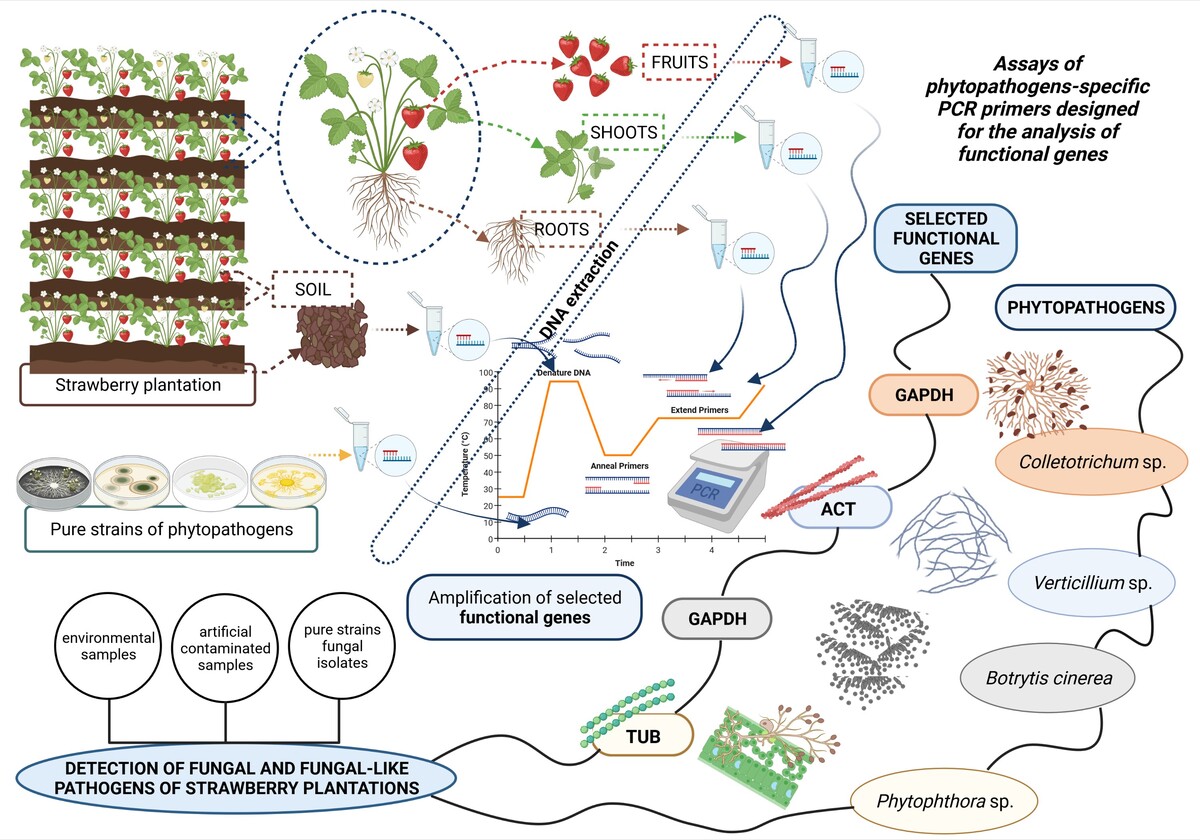
As the microbial quality of fresh fruits and food by-products is relevant, in this study we focus on the development of the detection methods of strawberry phytopathogens: Colletotrichum sp., Phytophthora sp., Verticillium sp., and Botrytis cinerea. The primers were designed based on β-tubulin for Phytophthora sp., actin for Verticillium sp., and glyceraldehyde-3-phosphate dehydrogenase for Colletotrichum sp. and B. cinerea. The primers were used successively on pure microbial strains, as well as on naturally and artificially contaminated soil and strawberry plant samples. The detection limit for artificially contaminated soil was as follows: B. cinerea (100-10 000 spores g-1 of soil), Colletotrichum sp. (1 000-100 000 spores g-1 of soil), and Verticillium sp. (100 000 spores g-1 of soil), whereas for artificially contaminated strawberry, it was the same for all tested pathogens (100 spores g-1 of strawberry). The same primers were used to test bio-preparations, reducing the presence of these potential phytopathogens within different strawberry plant cultivars. The results demonstrated that a developed assay using specific primers designed based on functional genes can be used as a molecular alternative for monitoring and routine investigation of samples contaminated by four important fungal and fungal-like strawberry plant pathogens, including assessing environmental samples such as soil and plants (roots, shoots, fruits).
FUNDING
This work was supported by The National Centre for Research and Development in the frame of the project BIOSTRATEG, contract number BIOSTRATEG3/344433/16/NCBR/2018.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES (48)
1.
Ann, P.J., 1994. Survey of soils suppressive to three species of Phytophthora in Taiwan. Soil Biol. Biochem. 26, 1239-1248. https://doi.org/10.1016/0038-0....
2.
Atallah, Z.K., Bae, J., Jansky, S.H., Rouse, D.I., Stevenson, W.R., 2007. Multiplex Real-time quantitative PCR to detect and quantify Verticillium dahliae colonization in potato lines that differ in response to Verticillium wilt. Phytopathology 97, 865-872. https://doi.org/10.1094/PHYTO-....
3.
Beckerman, J., Bessin, R., Welty, C., Athey, K., Wahle, E., Lewis, D., et al., 2022. Midwest Fruit Pest Management Guide 2023-2024.
4.
Black, J., Dean, T., Byfield, G., Foarde, K., Menetrez, M., 2013. Determining fungi rRNA copy number by PCR. J. Biomol. Tech. 24, 32-38. https://doi.org/10.7171/jbt.13....
5.
Bonants, P., Hagenaar-de Weerdt, M., Van Gent-Pelzer, M., Lacourt, I., Cooke, D., Duncan, J., 1997. Detection and identification of Phytophthora fragariae Hickman by the polymerase chain reaction. Eur. J. Plant Pathol. 103, 345-355. https://doi.org/10.1023/A:1008....
6.
Bragança, C.A.D., Damm, U., Baroncelli, R., Massola Júnior, N.S., Crous, P.W., 2016. Species of the Colletotrichum acutatum complex associated with anthracnose diseases of fruit in Brazil. Fungal Biol. 120, 547-561. https://doi.org/10.1016/j.funb....
7.
Colquhoun, J., Guedot, C., McManus, P., Knuteson, D., 2015. BioIPM Strawberry Workbook, BioIPM Strawberry Workbook. University of Wisconsin-Extension, Cooperative Extension.
8.
Camacho, C., Coulouris, G., Avagyan, V., Ma, N., Papadopoulos, J., Bealer, K., et al., 2009. BLAST+: Architecture and applications. BMC Bioinformatics 10, 421, https://doi.org/10.1186/1471-2....
9.
Dean, R., Van Kan, J.A.L., Pretorius, Z.A., Hammod-Kosack, K.E., Di Pietro, A., Spanu, P.D., et al., 2012. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13. https://doi.org/10.1111/j.1364....
10.
Debode, J., Van Hemelrijck, W., Baeyen, S., Creemers, P., Heungens, K., Maes, M., 2009. Quantitative detection and monitoring of Colletotrichum acutatum in strawberry leaves using real-time PCR. Plant Pathol. 58, 504-514. https://doi.org/10.1111/j.1365....
11.
Debode, J., van Poucke, K., França, S.C., Maes, M., Höfte, M., Heungens, K., 2011. Detection of multiple Verticillium species in soil using density flotation and real-time polymerase chain reaction. Plant Dis. 95, 1571-1580. https://doi.org/10.1094/PDIS-0....
12.
Diguta, C.F., Rousseaux, S., Weidmann, S., Bretin, N., Vincent, B., Guilloux-Benatier, M., et al., 2010. Development of a qPCR assay for specific quantification of Botrytis cinerea on grapes. FEMS Microbiol. Lett. 313, 81-87. https://doi.org/10.1111/j.1574....
13.
Drobek, M., Cybulska, J., Frąc, M., Pieczywek, P., Pertile, G., Chibrikov, V., et al., 2024. Microbial biostimulants affect the development of pathogenic microorganisms and the quality of fresh strawberries (Fragaria ananassa Duch.). Sci. Hortic. (Amsterdam). 327. https://doi.org/10.1016/j.scie....
14.
Duressa, D., Rauscher, G., Koike, S.T., Mou, B., Hayes, R.J., Maruthachalam, K., et al., 2012. A real-time PCR assay for detection and quantification of Verticillium dahliae in spinach seed. Phytopathology 102, 443-451. https://doi.org/10.1094/PHYTO-....
15.
Frąc, M., Hannula, S.E., Bełka, M., Jędryczka, M., 2018. Fungal biodiversity and their role in soil health. Front. Microbiol. 9, 1-9. https://doi.org/10,3389/fmicb.....
17.
Gu, Z., Eils, R., Schlersner, M., 2016. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847-2849. https://doi.org/10.1093/bioinf....
18.
Gu, Z., Gu, L., Eils, R., Schlesner, M., Brors, B., 2014. Circlize implements and enhances circular visualization in R. Bioinformatics 30, 2811-2812. https://doi.org/10.1093/bioinf....
19.
Hosoya, K., Nakayama, M., Matsuzawa, T., Imanishi, Y., Hitomi, J., Yaguchi, T., 2012. Risk analysis and development of a rapid method for identifying four species of Byssochlamys. Food Control 26, 169-173. https://doi.org/10.1016/j.food....
20.
Koprivica, M., Dulić-Marković, I., Jevtić, R., Cooke, D.E.L., 2009. Methods for detection of Phytophthora fragariae var. rubi on raspberry. Pestic. Fitomedicina 24, 177-184. https://doi.org/10.1007/978-1-....
21.
Kuchta, P., Jęcz, T., Korbin, M., 2008. The suitability of PCR-based techniques for detecting Verticillium dahliae in strawberry plants and soil. J. Fruit Ornam. Plant Res. 16, 295-304.
22.
Kumar, S., Stecher, G., Tamura, K., 2016. MEGA 7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870-1874. https://doi.org/10.1093/molbev....
23.
Lilja, A.T., Parikka, P.K., Pääskynkivi, E.A., Hantula, J.I., Vainio, E.J., Vartiamäki, H.A., et al., 2006. Phytophthora cactorum and Colletotrichum acutatum: survival and detection. Agric. Conspec. Sci. 71, 121-128.
24.
Lima, J.O., Pereira, J.F., Rincones, J., Barau, J.G., Araújo, E.F., Pereira, G.A.G., et al., 2009. The glyceraldehyde-3-phosphate dehydrogenase gene of Moniliophthora perniciosa, the causal agent of witches’ broom disease of Theobroma cacao. Genet. Mol. Biol. 32, 362-366. https://doi.org/10.1590/S1415-....
25.
Malarczyk, D., Panek, J., Frąc, M., 2019. Alternative molecular-based diagnostic methods of plant pathogenic fungi affecting berry crops – a review. Molecules 24. https://doi.org/10.3390/molecu....
26.
Malarczyk, D.G., Panek, J., Frąc, M., 2020. Triplex real-time pcr approach for the detection of crucial fungal berry pathogens – Botrytis spp., Colletotrichum spp. and Verticillium spp. Int. J. Mol. Sci. 21, 1-17. https://doi.org/10.3390/ijms21....
27.
Nahimana, A., Francioli, P., Blanc, D.S., Bille, J., Wakefield, A.E., Hauser, P.M., 2000. Determination of the copy number of the nuclear rDNA and beta-tubulin genes of Pneumocystis carinii f. sp. hominis using PCR multicompetitors. J. Eukaryot. Microbiol. 47, 368-372. https://doi.org/10.1111/j.1550....
28.
Nakayama, M., Hosoya, K., Matsuzawa, T., Hiro, Y., Sako, A., Tokuda, H., et al., 2010. A rapid method for identifying Byssochlamys and Hamigera. J. Food Prot. 73, 1486-1492. https://doi.org/10.4315/0362-0....
29.
Nakielska, M., Feledyn-Szewczyk, B., Berbeć, A.K., Frąc, M. Microbial biopreparations and their impact on organic strawberry (Fragaria x ananassa Duch.) yields and fungal infestation. Sustainability 2024, 16, 7559. https://doi.org/10.3390/su1617....
30.
Nazar, R.N., Hu, X., Schmidt, J., Culham, D., Robb, J., 1991. Potential use of PCR-amplified ribosomal intergenic sequences in the detection and differentiation of Verticillium wilt pathogens. Physiol. Mol. Plant Pathol. 39, 1-11. https://doi.org/10.1016/0885-5....
31.
Noh, Y.H., Lee, S., Whitaker, V.M., Cearley, K.R., Cha, J.S., 2017. A high-throughput marker-assisted selection system combining rapid DNA extraction high-resolution melting and simple sequence repeat analysis: Strawberry as a model for fruit crops. J. Berry Res. 7, 23-31. https://doi.org/10.3233/JBR-16....
32.
Oszust, K., Cybulska, J., Frąc, M., 2020. How do Trichoderma genus fungi win a nutritional competition battle against soft fruit pathogens? A report on niche overlap nutritional potentiates. Int. J. Mol. Sci. 21, 1-19. https://doi.org/10.3390/ijms21....
33.
Panek, J., Frąc, M., 2018. Development of a qPCR assay for the detection of heat-resistant Talaromyces flavus. Int. J. Food Microbiol. 270, 44-51. https://doi.org/10.1016/j.ijfo....
34.
Parikka, P., 2003. Susceptibility of strawberry varieties to crown rot (Phytophthora cactorum) in greebhouse tests. Acta Hortic. 626, 138-189. https://doi.org/10.17660/ActaH....
35.
Peres, N.A., Timmer, L.W., Adaskaveg, J.E., Correll, J.C., 2005. Lifestyles of Colletotrichum acutatum. Plant Dis. 89, 784-796. https://doi.org/10.1094/PD-89-....
36.
Pertile, G., Frąc, M., Fornal, E., Oszust, K., Gryta, A., Yaguchi, T., 2020. Molecular and metabolic strategies for postharvest detection of heat-resistant fungus Neosartorya fischeri and its discrimination from Aspergillus fumigantus. Postharvest Biol. Technol. 161. https://doi.org/10.1016/j.post....
37.
Pertile, G., Panek, J., Oszust, K., Siczek, A., Frąc, M., 2018. Intraspecific functional and genetic diversity of Petriella setifera. PeerJ 6, 1-24. https://doi.org/10.7717/peerj.....
38.
Prigigallo, M.I., Mosca, S., Cacciola, S.O., Cooke, D.E.L., Schena, L., 2015. Molecular analysis of Phytophthora diversity in nursery-grown ornamental and fruit plants. Plant Pathol. 64, 1308-1319. https://doi.org/10.1111/ppa.12....
39.
Rigotti, S., Gindro, K., Richter, H., Viret, O., 2002. Characterization of molecular markers for specific and sensitive detection of Botrytis cinerea Pers.: Fr. In strawberry (Fragaria×ananassa Duch.) using PCR. FEMS Microbiol. Lett. 209, 169-174. https://doi.org/10.1016/S0378-....
40.
Schlenzing, A., 2009. Identification of Phytophthora fragariae var. rubi by PCR, in: Burns, R. (Ed.), Plant Pathology: Techniques and Protocols. 161-169. https://doi.org/10.1007/978-1-....
41.
Schrader, C., Schielke, A., Ellerbroek, L., Johne, R., 2012. PCR inhibitors - occurrence, properties and removal. J. Appl. Microbiol. 113, 1014-1026. https://doi.org/10.1111/j.1365....
42.
Sreenivasaprasad, S., Talhinhas, P., 2005. Genotypic and phenotypic diversity in Colletotrichum acutatum, a cosmopolitan pathogen causing anthracnose on a wide range of hosts. Mol. Plant Pathol. 6, 361-378. https://doi.org/10.1111/j.1364....
43.
Talhinhas, P., Sreenivasaprasad, S., Neves-Martins, J., Oliveira, H., 2005. Molecular and phenotypic analyses reveal association of diverse Colletotrichum acutatum groups and a low level of C. gloeosporioides with olive anthracnose. Appl. Environ. Microbioloy 71, 2987-2998. https://doi.org/10.1128/AEM.71....
44.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., Kumar, S., 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870-1874.
45.
Trzciński, P., Frąc, M., Lisek, A., Przybył, M., Frąc, M., Sas-Paszt, L., 2021. Growth promotion of raspberry and strawberry plants by bacterial inoculants. Acta Sci. Pol. Hortorum Cultus 20, 71-82. https://doi.org/10.24326/ASPHC....
46.
Wei, T., Lu, G., Clover, G., 2008. Novel approaches to mitigate primer interaction and eliminate inhibitors in multiplex PCR, demonstrated using an assay for detection of three strawberry virus. J. Virol. Methods 151, 132-139. https://doi.org/10.1016/j.jvir.... Epub 2008 May 1.
47.
Yaguchi, T., Imanishi, Y., Matsuzawa, T., Hosoya, K., Hitomi, J., Nakayama, M., 2012. Method for identifying heat-resistant fungi of the genus Neosartorya. J. Food Prot. 75, 1806-1813. https://doi.org/10.4315/0362-0....
48.
Zan, K., 1962. Activity of Phytophthora infestans in soil in relation to tuber infection. Trans. Br. Mycol. Soc. 45, 205-221. https://doi.org/10.1016/S0007-....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.